In the modern periodic table, the elements are listed in order of increasing atomic number. The atomic number is the number of protons in the nucleus of an atom. The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present). The number of protons determines how many electrons surround the nucleus, and it is the arrangement of these electrons that determines most of the chemical behavior of an element.
In a periodic table arranged in order of increasing atomic number, elements having similar chemical properties naturally line up in the same column (group). For instance, all of the elements in Group 1A are relatively soft metals, react violently with water, and form 1+ charges; all of the elements in Group 8A are unreactive, monatomic gases at room temperature, etc. In other words, there is a periodic repetition of the properties of the chemical elements with increasing mass.
In the original periodic table published by Dimitri Mendeleev in 1869, the elements were arranged according to increasing atomic mass— at that time, the nucleus had not yet been discovered, and there was no understanding at all of the interior structure of the atom, so atomic mass was the only guide to use. Once the structure of the nucleus was understood, it became clear that it was the atomic number that governed the properties of the elements.


The Atomic Number For Nitrogen-15
The atomic number of nitrogen is 7. How many protons, neutrons, and electrons make up an atom of nitrogen-15?

Atomic Mass For Nitrogen
1 Answer
- 14.007 is the average atomic mass of all natural Nitrogen Isotopes. 14.007 is so close to 14 because so much of naturally occurring nitrogen is Nitrogen-14. Nitrogen-14 (or N-14) has 14 Nucleons Nitrogen-14 is an isotope with 14 nucleons Nitrogen has an atomic number of 7 which means 7 protons.
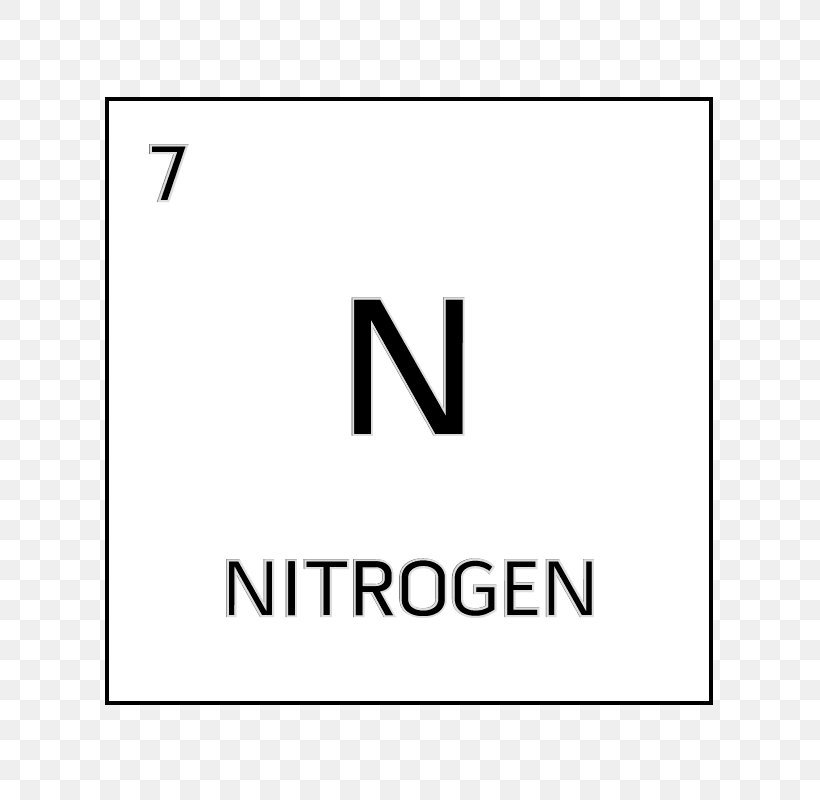
- Nitrogen is a chemical element with symbol N and atomic number 7. It is the lightest pnictogen and at room temperature, it is a transparent, odorless diatomic gas. Nitrogen is a common element in the universe, estimated at about seventh in total abundance in the Milky Way and the Solar System.
By definition, if
Explanation:
What Is Atomic Number For Nitrogen
The atomic number is the number of protons in the nucleus of an atom. The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present). The number of protons determines how many electrons surround the nucleus, and it is the arrangement of these electrons that. Nitrogen, nonmetallic element of Group 15 Va of the periodic table. It is a colorless, odorless, tasteless gas that is the most plentiful element in Earth’s atmosphere and is a constituent of all living matter. Its atomic number is 7 and it is denoted by the symbol ‘N’ in the periodic table.
The atomic number is by definition the number of protons, positively charged particles, contained within the atomic nucleus. So if there are 7 protons, there MUST be 7 electrons, 7 negatively charged particles. Why? Because matter is electrically neutral, and positive and negative particles must be equal.
Electrons have negligible mass; the mass number depends on the number of protons contained within the nucleus, PLUS the number of neutrons. Given that we have
The Atomic Number For Nitrogen

The Atomic Number For Nitrogen Is 14
Related questions
